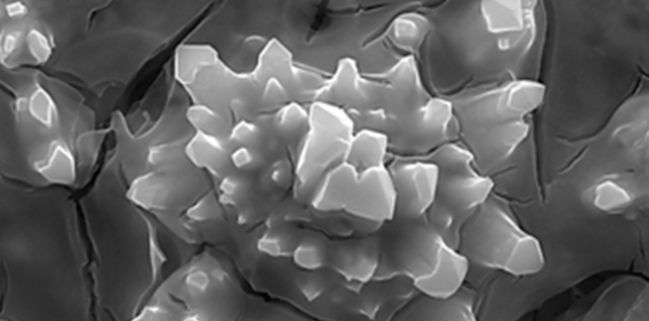
Good surface coverage
Chlor Alkali (CA) and Alkaline Water Electrolysis (AWE) cathodes characteristics:
Cathode electrodes used in chlor-alkali electrolyzers are usually made of carbon steel in diaphragm cells and nickel in membrane cells.
AWE cells use a diaphragm as separator but with a configuration like CA membrane devices and cathodes are made in nickel or nickel - plated steel.
In this operations, the cathode overvoltage, influenced mainly by the electrode material and the operating current density, can heavily impact the overall the cell voltage and therefore the specific power consumption of the plant.